Together We Can Advance Research
The Hypothalamic-Pituitary Brain Tumors Patient Registry, established in 2024 by RAWF in collaboration with international experts and stakeholders within the hypothalamic-pituitary brain tumor community, serves as a pivotal resource for comprehensive data collection and analysis. Our mission is to enhance understanding of the natural history, varied characteristics, and associated co-morbidities of hypothalamic-pituitary brain tumors, while facilitating clinical trial recruitment, and illuminating the patient and caregiver journey. Additionally, the Registry aims to identify crucial areas for future research and treatment development.
Why Participate
Contribute to Research
Your participation directly contributes to advancing knowledge and treatment options for hypothalamic-pituitary brain tumors.
Global Collaboration
Join a global effort to pool resources and expertise in tackling this rare condition, fostering collaboration among patients, caregivers, researchers, and clinicians
Impact Patient Outcomes
Help researchers and clinicians better understand the disease, leading to improved outcomes and quality of life for patients.
Shape Future Therapies
Your insights shape the development of new therapies and interventions, potentially benefiting countless individuals affected by these tumors.
Empowerment
By sharing your experiences and insights, you empower yourself and others within the hypothalamic-pituitary brain tumor community to advocate for better care and support.
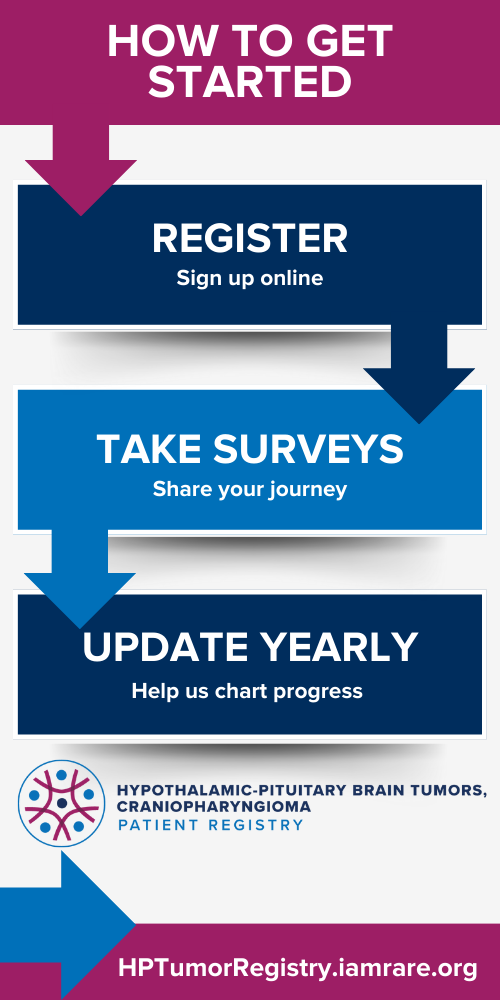
How to Participate
- Follow the link to register on the National Organization for Rare Disorders Registry platform.
- Complete the surveys covering aspects such as diagnosis, medical history, health challenges, medications, nonmedical interventions, hypothalamic obesity, and hunger behavior.
- Keep an eye out for new survey notifications and promptly respond to them.
- Update your information annually to ensure the data remains current and relevant.
How Participation Helps Research
Active participation in the Hypothalamic-Pituitary Brain Tumors Patient Registry significantly contributes to advancing research in this field.
- By sharing your valuable insights and data, you directly contribute to collaborative research endeavors with academic and industry partners.
- Your contributions enable researchers to conduct retrospective studies and clinical trials on new therapies, ultimately leading to improved treatment options and outcomes for individuals affected by hypothalamic-pituitary brain tumors.
- De-identified, aggregated data from the Registry serve as a valuable resource for uncovering insights into disease progression, treatment effectiveness, and identifying areas for further investigation.
- Access to this data is governed by stringent guidelines set forth by the Hypothalamic-Pituitary Brain Tumor Patient Registry Advisory Board, ensuring ethical oversight and data protection.
- Through your participation, you play a crucial role in driving forward research efforts aimed at enhancing our understanding and management of hypothalamic-pituitary brain tumors.
Data Privacy FAQs
The Hypothalamic-Pituitary Brain Tumors Patient Registry is a secure platform hosted on the National Organization for Rare Disorders (NORD) platform. It follows strict government guidelines to assure patient information is protected. The platform is compliant with:
• US Health Information Privacy Laws (HIPPA, HITECH, and FISMA)
• State privacy laws (where applicable)
• FDA regulations on electronic records (21CFR Part 11)
• European Union Data Directive and General Data Protection Regulation
NORD launched its first patient registry in 2014 and is currently hosting over 60 registries. Since its inception, there has never been a data breach or an incident where participant data security was compromised to date.
The Hypothalamic-Pituitary Brain Tumors Patient Registry protocol and procedures have been reviewed and approved by an Institutional Regulatory Board (IRB) (North Star Review Board), which has ensured that human subject research is conducted in accordance with all federal, institutional, and ethical guidelines.
The platform is served over HTTPS, which means that the data is encrypted when being sent from the user’s browser to the NORD servers. The data is also kept encrypted in the NORD database (data at rest). Communications between the registry platform application server and the database are also encrypted. As with any information one provides electronically, there is a very rare chance that privacy could be compromised. However, the registry and the security measures minimize the chance of this occurring.
Further, the information participants provide is de-identified, which means that all Personal Health Identification (PHI) has been removed, and then aggregated (combined and summarized) with data from other registry participants. The registry usually shares only de-identified data with the research community conducting research studies or clinical trials, companies developing potential drugs or other treatments for hypothalamic-pituitary brain tumors, or other parties. In some cases that will be reviewed by the registry advisory board, data containing PHI will be shared if the research cannot be done without it. All data sharing is in accordance with the data access and sharing guidelines outlined in the IRB-approved registry protocol. Any information that identifies participants is removed. The only people with direct access to the registry data are the investigators on the registry protocol.
The Health Insurance Portability and Accountability Act (HIPAA) of 1996 is the primary law that oversees the use of, access to and disclosure of Protected health information (PHI), also referred to as personal health information, in the United States.
HIPAA defines PHI as data that relates to the past, present or future health of an individual; the provision of healthcare to an individual; or the payment for the provision of healthcare to an individual. HIPAA regulates how this data is created, collected, transmitted, maintained and stored by any HIPAA-covered organization.
HIPAA lists different information identifiers that, when paired with health information, become PHI. Some of these identifiers on their own can allow an individual to be identified, contacted or located. Others must be combined with other information to identify a person. The registry uses a number of PHI that includes the following (list non exhaustive):
1. First and last name;
2. Date of Birth (DOB)
3. Sex recorded on birth certificate
4. City/Municipality of Birth
5. State/Province of residence
6. Email address
7. Diagnoses
Although there is a very rare chance that privacy could be compromised, it is important to enter accurate information. The consent form that you must read and sign to participate in the study is a legal document, and it must be signed with your real name for it to be valid. Also, for research purposes, it is very important that the data contained in the registry is trustworthy so that the voice of patients and caregivers is credible and can be heard by the community of healthcare providers, scientists, regulatory agencies. Additionally, entering incorrect information such as DOB will jeopardize the creation of an identification number such as CRID or GUID that can be used to link your medical information across different databases without sharing personal health information.
NORD stores Sponsor and Participant Registry Data on NORD encrypted servers and/or encrypted servers of third-party vendors hosted in Canada. Regular back-up at commercially acceptable intervals is provided. These servers meet industry standards and are compliant with US and international regulations, including GDPR.
The study data are owned by the study sponsor, the Raymond A. Wood Foundation (RAWF). RAWF decides how and with whom to share the data. NORD staff will have access to the data for activities related to support and maintenance of the Platform and will collect Platform-wide participation statistics. The specifics will be outlined in your informed consent.
All data, including those with PHI, will be stored in a password protected secure server. Access to PHI will be limited to:
• Approved members of the Hypothalamic-Pituitary Brain Tumors Patient Registry research team
• NORD staff, in cases where technical support is needed and with the permission of registry staff
• With agreement from the Sponsor, NORD may conduct IRB-approved, cross-disease research using registry data.
In all cases, your privacy will be protected. The Registry Advisory Board will evaluate all requests for data from researchers. Researchers will only be provided with the minimum data necessary to accomplish their research study goals. Data containing PHI will only be shared if the research cannot be done without it. The researchers will be required to sign a Confidentiality Agreement in which they promise to keep your information safe.
For individuals living outside the United States who choose to share information about themselves, the same protections for privacy and confidentiality are offered as in the United States. Residents of the European Union and Switzerland have additional particular rights related to personal information. This information is disclosed within the informed consent document. If an individual signs this document, they acknowledge that they are disclosing information that would otherwise be private. Privacy laws in an individual’s country may have different protections than those provided in the United States.
Registry participants who are residents of the European Union and Switzerland are entitled to:
• Request to obtain access to and rectification or erasure of personal data;
• Receive personal data in a portable, readily-accessible format;
• Restrict or withdraw permission for the processing of personal information; and
• Lodge a complaint with an appropriate supervisory authority.

Together, we can make a difference in the lives of those living with hypothalamic-pituitary brain tumors.
Joining the Hypothalamic-Pituitary Brain Tumors Patient Registry provides a unique opportunity for patients and caregivers to actively contribute to the advancement of knowledge and treatment options for this rare condition. By sharing your experiences and insights, you become an integral part of a global effort to improve outcomes and quality of life for individuals affected by hypothalamic-pituitary brain tumors. Your participation not only helps researchers and clinicians better understand the disease but also directly impacts the development of new therapies and interventions.
Join us in our mission. Register today.
To receive regular updates on Registry data, please email us at registry@rawoodfoundation.org.
Access downloads of IRB approved Hypothalamic-Pituitary Brain Tumors Patient Registry Protocol and Informed Consent documents [LINK].
For more information, contact Nathalie Kayadjanian, Ph.D., Scientific Advisor, or the registry coordinator at registry@rawoodfoundation.org.
Third parties seeking to recruit participants for research studies through the Registry must demonstrate IRB approval of their respective studies. For further inquiries or information, please contact us at registry@rawoodfoundation.org.




